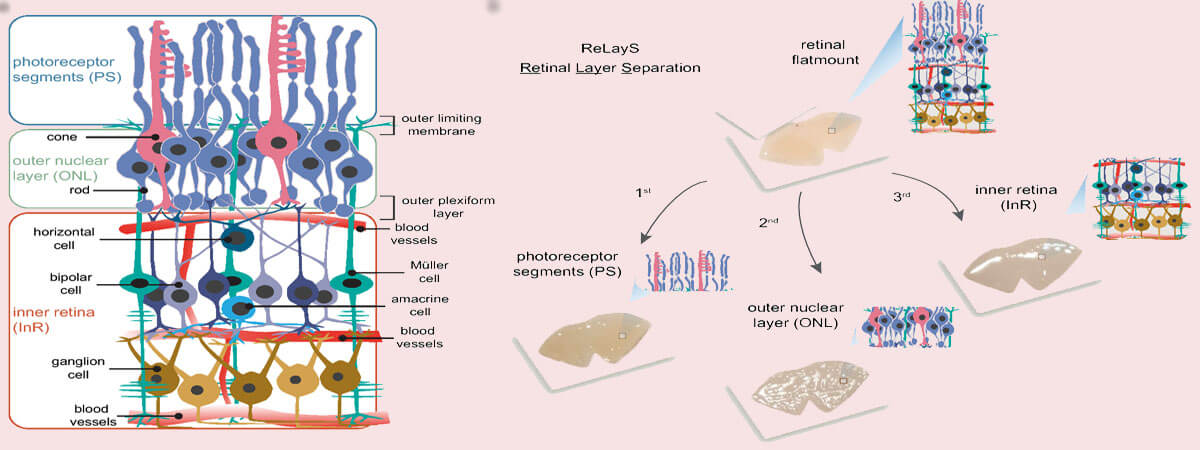
Understanding the physiology of the retina, and especially of the highly polarized photoreceptors, is essential not only to broaden our knowledge of the processes required for normal vision, but also to develop effective therapies to prevent or slow retinal degenerative diseases. However, the molecular analysis of photoreceptors is a challenge due to the heterogeneity of the retinal tissue and the lack of easy and reliable methods for cell separation. Here we present the ReLayS method—a simple technique for the separation of photoreceptor segments (PS) containing both inner and outer segments, outer nuclear layer (ONL), and inner retina (InR) that contains the remaining retinal layers. The layer-specific material isolated from a mouse half-retina with the ReLayS method was sufficient for protein isolation and Western blotting or RNA isolation and real-time PCR studies. The separation of PS, ONL, and InR was successfully validated by Western blotting and real-time PCR using proteins and genes with known expression profiles within the retina. Furthermore, the separation of the PS from the ONL enabled the detection of light-driven translocation of transducin from the PS to the soma. ReLayS is a simple and useful method to address protein and possibly metabolites distribution in photoreceptor compartments in various situations including development, ageing, and degenerative diseases.
The retina is a highly heterogeneous tissue with seven major cell types arranged in three cellular layers connected by two synaptic layers, providing visual input detection and initial signal processing1 (Fig. 1a). The outermost cell layer is populated by rod and cone photoreceptors. Photoreceptors are highly polarized cells consisting of a light-sensitive outer segment (OS), an inner segment (IS) containing the metabolic machinery of the cell, a soma with the nucleus in the outer nuclear layer (ONL), and an axon ending in a synaptic terminal in the outer plexiform layer (OPL). In the OPL, photoreceptors connect to the second-order neurons of the inner retina, which itself is morphologically and functionally organized in several layers2. The inner nuclear layer (INL) contains cell bodies of bipolar, horizontal, amacrine, and Müller glial cells. Neurotransmission from second-order neurons in the INL to retinal ganglion cells occurs in the inner plexiform layer (IPL). The ganglion cell bodies populate the ganglion cell layer (GCL) together with some amacrine cells and astrocytes. Microglia localize to the inner retina, but can migrate to the outer retina upon activation by photoreceptor damage or stress.
Retinal pathologies associated with photoreceptor degeneration such as age-related macular degeneration, diabetic retinopathy, and retinitis pigmentosa comprise a large proportion of untreatable blindness globally3. To understand the underlying pathological mechanisms and develop successful therapies to preserve vision, it is essential to study the biochemical and molecular events in photoreceptors, ideally on a subcellular level. However, isolation of photoreceptor inner and outer segments as well as of photoreceptor cell bodies for biochemical and molecular analysis is a major challenge. The most widely used method for isolation of photoreceptor outer segments was originally developed for bovine retinas4 but has been adapted for other eyes including those of pigs5, amphibians6, and dogs7. In this method, the tissue is mechanically ruptured and the outer segments are purified by a sucrose gradient4. However, this method has two major limitations. First, it requires a sizable amount of retinal tissue, and second, inner segments and photoreceptor cell bodies are lost in the procedure. Other methods for the isolation of retinal cells are based on tissue dissociation8,9. In most instances, those methods also require pooling of several retinas and involve rather complicated and long-lasting procedures, including manual sorting based on cell morphology or flow cytometry. Laser capture microdissection is an alternative technique for the isolation of small tissue samples and is compatible with mass spectrometry applications as well as DNA and RNA profiling10,11. Unfortunately, the limiting amount of the samples as well as the tissue processing makes it incompatible with molecular analysis such as Western blotting and metabolite detection assays. Serial tangential sectioning of flat-mounted frozen retinas is a method well suited for protein analysis using Western blotting12. This method depends on the perfect alignment of the retinal layers with the cutting plane of the cryostat knife to collect similar fractions from different retinas. Because the collection of many fractions from a single retina is possible by this method, it is well suited for high resolution localization studies. Highly expressed proteins can be detected by Western blotting, proteins with reduced expression may need more sensitive methods such as mass spectrometry. Two peeling methods were introduced by Rose et al. for the isolation of photoreceptor outer and/or inner segments from the mouse retina13. The first method requires lyophilization of the retina and uses Scotch tape for peeling the photoreceptor outer and inner segments. The second method uses Whatman filter paper to separate the photoreceptor outer segments from retinal tissue. Isolation of the ONL is, however, not provided in those methods.
Here we introduce a simple method for retinal layer separation (ReLayS) from mouse eyes that efficiently and reproducibly isolates three fractions that contain the photoreceptor inner and outer segments (PS), the outer nuclear layer (ONL), and the inner retina (InR) with all other layers. Samples prepared with the ReLayS method from individual half-retinas provide sufficient material for Western blotting or real-time PCR, allowing the study of the subcellular molecular composition of photoreceptors. The ReLayS method was validated by Western blotting and quantitative RT-PCR using several representative proteins and genes for photoreceptor compartments and different retinal cell types.
https://www.nature.com/articles/s41598-022-24586-8